
In this article, the author has explained what is evaporation, the characteristics of evaporation, and factors such as temperature, surface area, the strength of intermolecular forces, and size of molecules, affecting the rate of evaporation.
Table of Contents
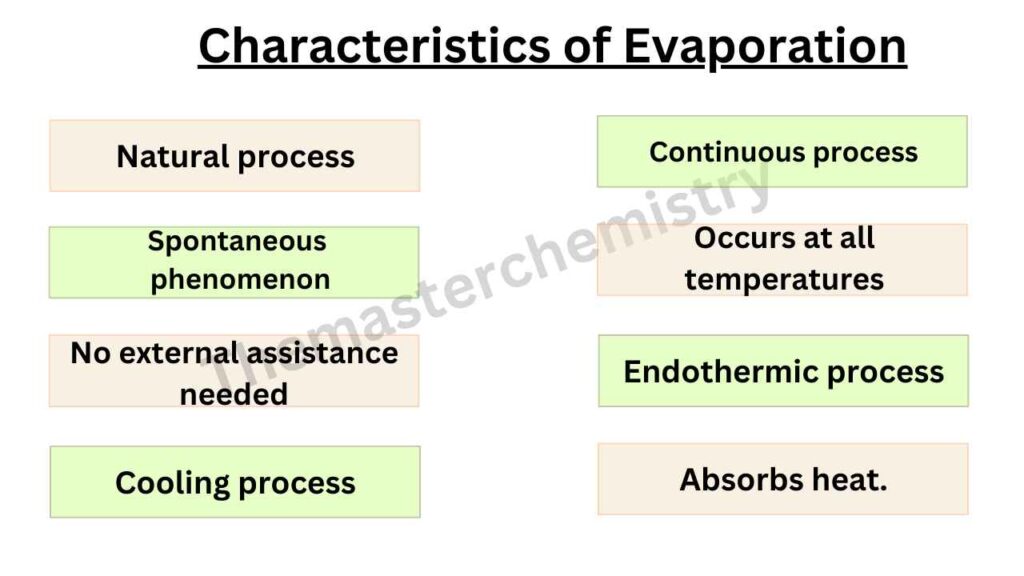
The process of spontaneous change of a liquid into vapors is known as evaporation.
H2O ——> H2O g H2 = + 40.7 Kj/mol

It is always a continuous process at all temperatures from the hottest deserts to the coldest Antarctic region.
According to kinetic molecular theory, molecules of liquid possess kinetic energy. All the molecules do not have the same Kinetic energy.
Some molecules have higher kinetic energy than the average value. When such types of molecules come to the surface of the liquid, these overcome the intermolecular force and thus escape from the surface of the liquid as vapors. This is called evaporation.
This energy that overcomes the intermolecular forces may result from collisions of molecules or external heat sources.
In an open container, at constant temperature evaporation continues at the same rate until all the liquid is converted into vapor.
Watch the video lecture for a better understanding of Evaporation.
When we talk about evaporation, we particulary talk about liquid substances. Now we observe different types of liquids that takes different time to fully evaporate. For example petrol evaporates faster than water. Similarly organic solvents such as methanol, hexane, ethyl acetate also evaporates faster than water at room temperature.
It means there are some factors involved in evaporation.
The rate of evaporation of a liquid depends upon the following factors.

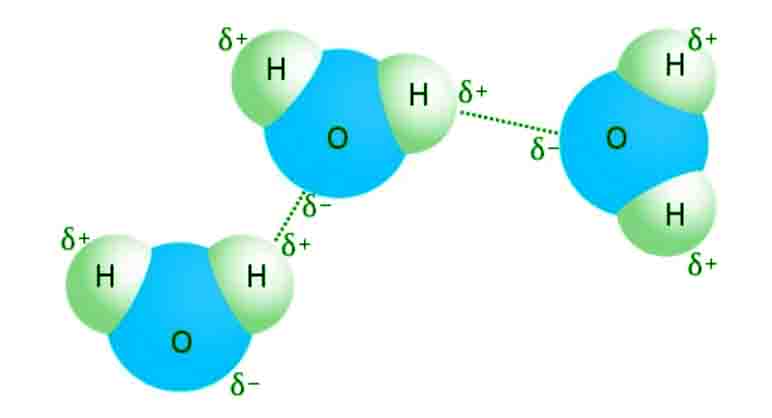
We know that molecules in liquids are held together through intermolecular forces. Due to varied atomic size different liquids evaporates at a different rate.
If the intermolecular forces are stronger than the rate of evaporation will be lower.
If the intermolecular forces are weaker than the rate of evaporation will be higher.
The reason is that in case of strong inter and intramolecular forces the molecules will have more potential energy hence lower rate of evaporation and vice versa.
Let us take an example of water and ether.
The rate of evaporation of water is lower than ether. Because in the water there is strong hydrogen bonding present within the water molecules.
Temperature is another very important factor that determines the rate of evaporation of liquids. Temperature and intermolecular are closely related thermodynamically.
Increase in temperature increase the rate of evaporation and vice vera.
The reason is that when temperature increases, the kinetic energy of molecules also increase than average. Therefore, more molecules are escaped from the surface of liquid and rate of evaporation increases.
Let us understand this with the help of a simple example.
Consider two pools one in a desert and other in Antarctic region.
Which pool’s water will evaporate faster? Definitely the one in desert because the temperature is higher in desert as compared to Antarctic region.
It is referred as the total area exposed to open environment. Larger the surface are, higher is the rate of evaporation and vice versa.
It is because with a larger surface area more number of molecules escape. That is why expanded clothes are dried earlier than the unexpanded.
Similarly if we fill one liter of water in two pots where one is open and other is narrow. The rate of evaporation will be faster in case of pot with wider shape because of larger surface area.
Larger the size of molecules of a liquid lower will be the rate of evaporation.
The reason is that larger molecules develop stronger intermolecular forces that cause a decrease in the rate of evaporation. This is the reason nitrogen evaporates faster than oxygen.
Water is a polar liquid and due to strong hydrogen bonding high energy is required to separate the molecules from each other at its boiling point. Ch4 is a non polar in nature and has weak London dispersion forces.
No, doubt the temperature of boiling water and steam is same but the heat content of steam is greater than that of boiling water. The latent heat of vaporization is responsible for sever burns.
Body is warmer as compared to water. Water evaporates from the body due to high temperature of the body and larger surface area. Evaporation for the surface of body becomes fast. Evaporation needs energy which is supplied by the body which gets a sense of cooling.
Earthenware vessels are porous. Water molecules come out from these pores and evaporate. These molecules of water need the energy to overcome intermolecular attractive forces. They get this energy from other molecules of water. Thus the temperature of the remaining water decreases.
High energy molecules escape and change into vapors during evaporation. So, the temperature of the liquid falls. To compensate for that, the heat flows from the surrounding to the region of lower temperature. This causes the temperature of the surroundings to decrease. Therefore, the evaporation of liquids causes cooling.
